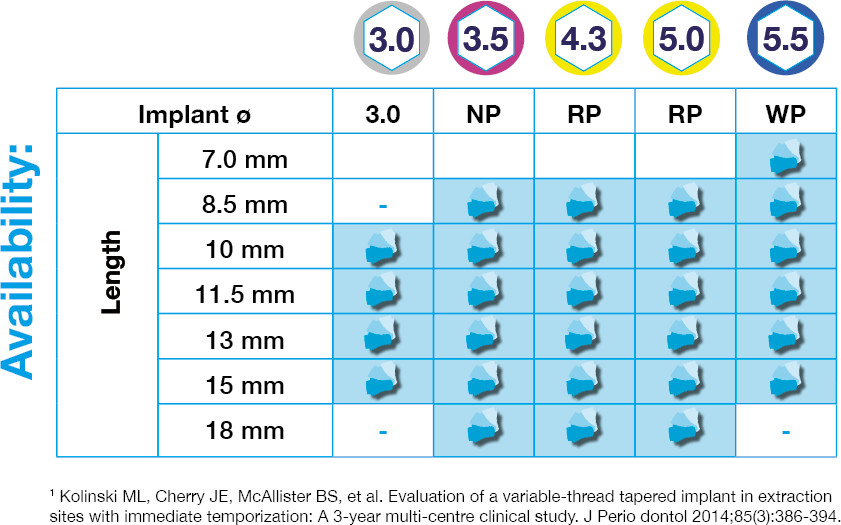
DESS® Active Hex Implant is the first Zero Waste Implant with infinite packaging on the market.


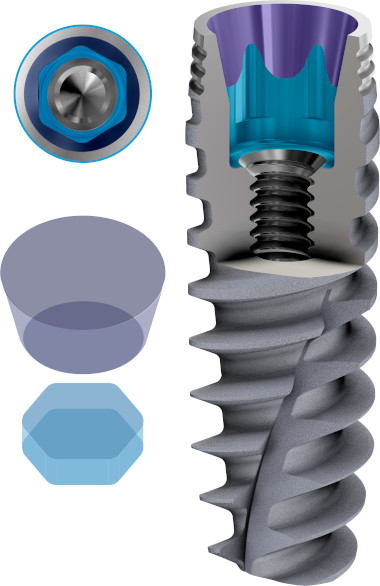
The Connection of Active Hex implants
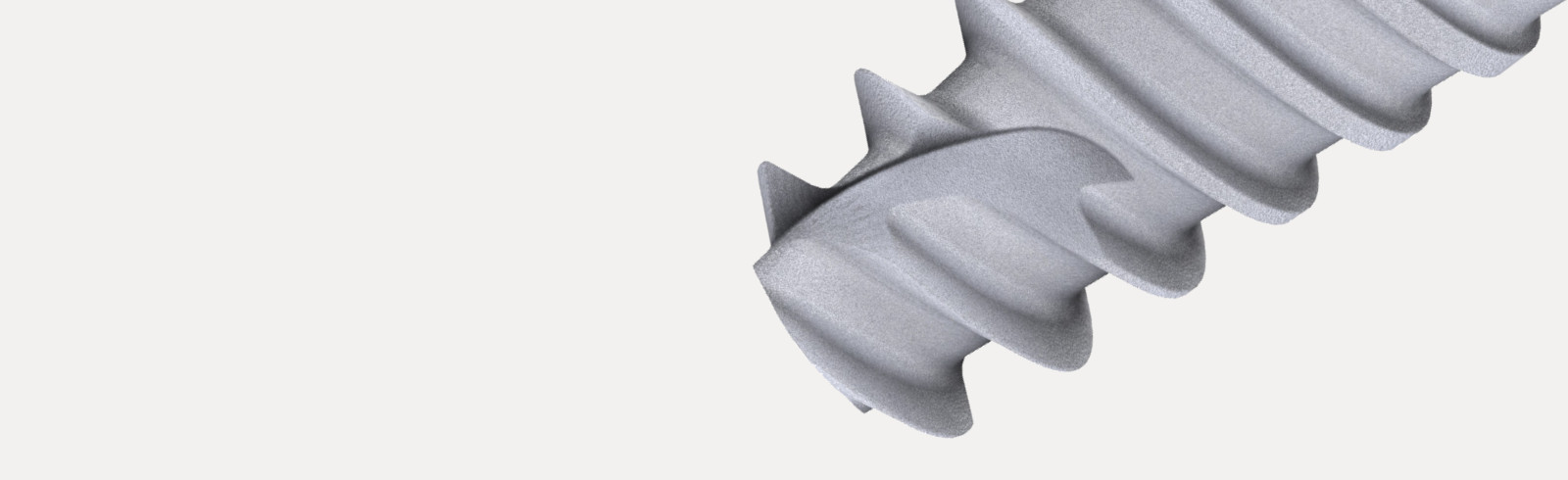
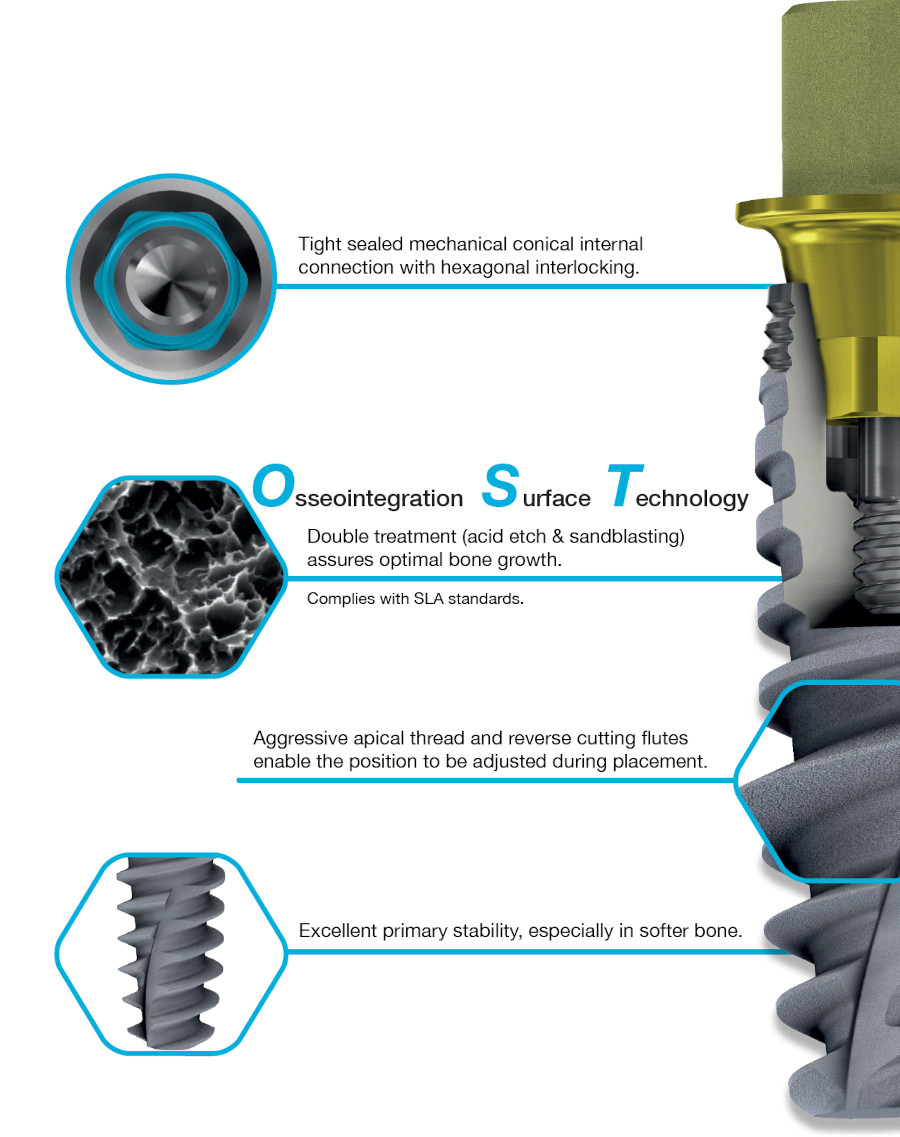
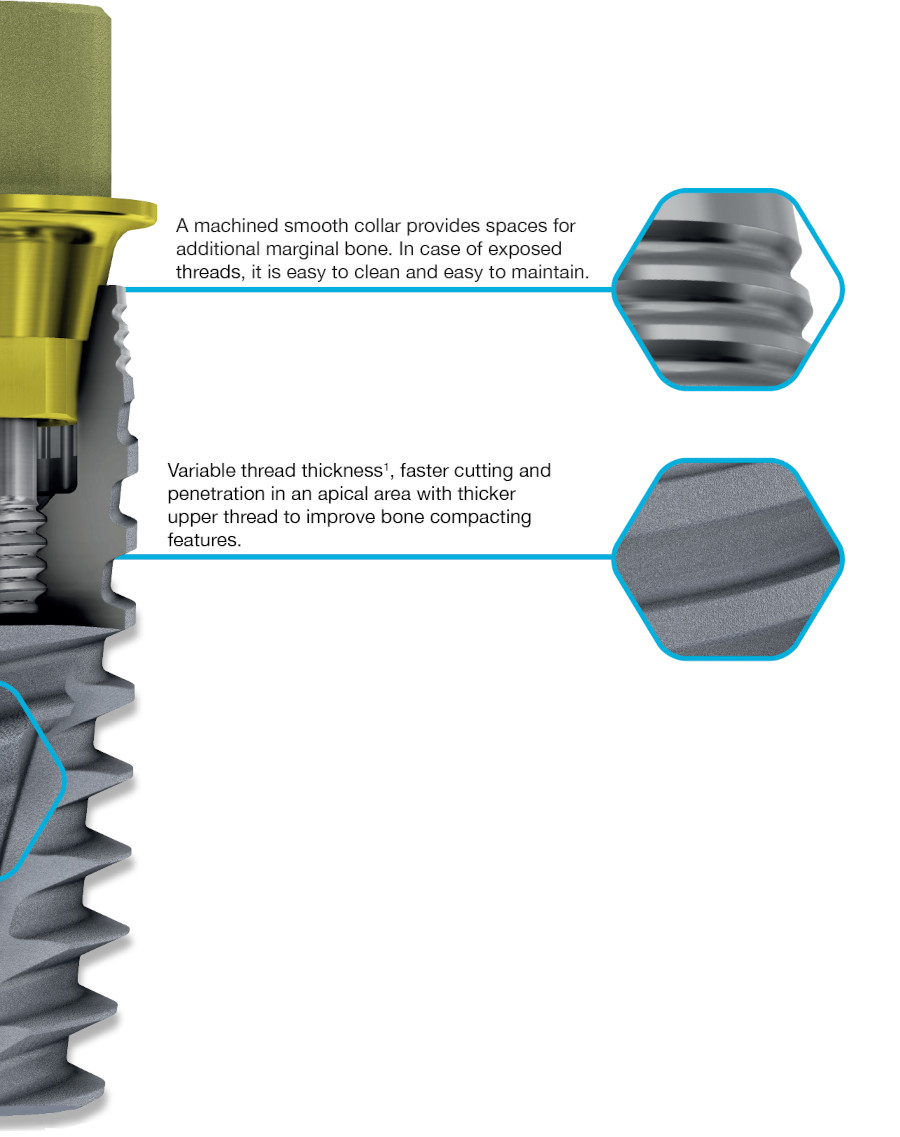
Dual Function internal conical connection, at 12º creating the ultimate seal whilst reducing the possibility of micromovements, with hexagonal interlocking (6 positions) for a firmer connection with superior mechanical strength.
The Zero Waste Implant
We recycle and re-use 100% of its packaging.
- 100% Recycled cardboard box
- Widely Recyclable PET blister
- Titanium reusable/recyclable vial
Collaborate with us during the packaging recycling process and every 10 packs returned; we will be giving you 1 free implant.
Advantages of DESS implants

The first eco-friendly implant on the market Help us recycle the packaging and prevent it from damaging our planet..

Osseointegration Surface Technology: Double treatment (acid etch & sandblasting) assures optimal bone growth.

Lifetime Warranty on all our prosthetic abutments and dental implants.

Internal conical connection with a dual function, at 12º creating the ultimate seal whilst reducing the possibility of micromovements, with hexagonal interlocking for a firmer connection with superior mechanical strength.
ACTIVE HEX IMPLANT CATALOGUE
Discover all the details and advantages of the Active Hex implant system.
Download catalogue

ACTIVE HEX SURGICAL KIT
Designed to be simple and easy to use
The DESS® Active HEX surgical kit can be customised to the different protocols arranging the drills as per the case needs. With flush silicone inserts that are easily wiped clean and manufactured in autoclavable material, the DESS® surgical kit is easy to keep clean and sterilised.
If you already have a kit for this type of connection, there is no need to acquire the DESS® surgical kit, our implants are 100% compatible with the kits of the brand of reference.
Prosthetic abutments for Active Hex implants
If you need more information about DESS dental implants, prosthetic abutments, or drilling protocols, please contact your local supplier.